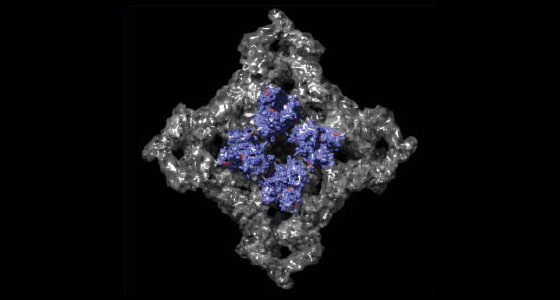
The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule
Authors: Ching-Chieh Tung, Paolo A. Lobo, Lynn Kimlicka, Filip Van Petegem. Lab of Filip Van Petegem, Cardiovascular Research Group, Department of Biochemistry and Molecular Biology.
Published in Nature 468, 585-588, November 25, 2010. doi:10.1038/nature09471
Abstract: Many physiological events require transient increases in cytosolic Ca2+ concentrations. Ryanodine receptors (RyRs) are ion channels that govern the release of Ca2+ from the endoplasmic and sarcoplasmic reticulum1. Mutations in RyRs can lead to severe genetic conditions that affect both cardiac and skeletal muscle, but locating the mutated residues in the full-length channel structure has been difficult2, 3. Here we show the 2.5 Å resolution crystal structure of a region spanning three domains of RyR type 1 (RyR1), encompassing amino acid residues 1–559. The domains interact with each other through a predominantly hydrophilic interface. Docking in RyR1 electron microscopy maps4, 5 unambiguously places the domains in the cytoplasmic portion of the channel, forming a 240-kDa cytoplasmic vestibule around the four-fold symmetry axis. We pinpoint the exact locations of more than 50 disease-associated mutations in full-length RyR1 and RyR2. The mutations can be classified into three groups: those that destabilize the interfaces between the three amino-terminal domains, disturb the folding of individual domains or affect one of six interfaces with other parts of the receptor. We propose a model whereby the opening of a RyR coincides with allosterically coupled motions within the N-terminal domains. This process can be affected by mutations that target various interfaces within and across subunits. The crystal structure provides a framework to understand the many disease-associated mutations in RyRs that have been studied using functional methods, and will be useful for developing new strategies to modulate RyR function in disease states.